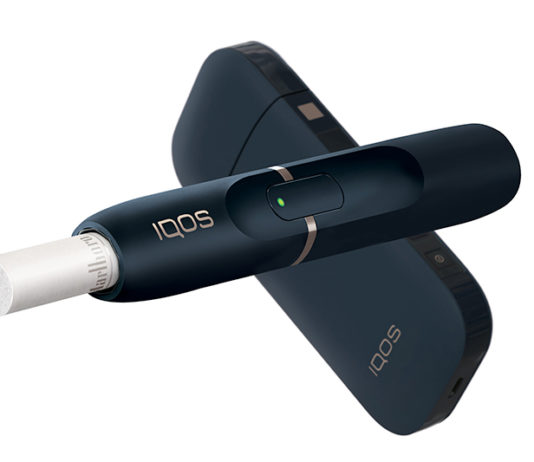
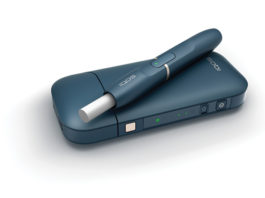
FDA Authorizes Philip Morris USA to Sell IQOS in U.S.
Altria Group, Inc. has revealed that it has received authorization from the U.S. Food and Drug Administration (FDA) to sell its heat-not-burn device, IQOS,...
Altria Invests More in Reduced Harm Tobacco Product Category
Altria has long been known for its traditional cigarette products but the company's new focus could make it a leader in the reduced harm...
British American Tobacco Releases 2018 Harm Reduction Focus Report
Harm reduction has been a major initiative and focus of British American Tobacco (BAT) in recent years and 2018 was no different. With the...
Philip Morris International to Regain Full U.S. Commercial Rights to IQOS
Altria has reached an agreement with Philip Morris International (PMI) relating to the IQOS Tobacco Heating System. Altria has revealed that it will receive...
FDA Authorizes IQOS to be Marketed as Modified Risk Tobacco Product
The U.S. Food and Drug Administration (FDA) has given Altria Group the ok to market its heat not burn device, IQOS, as a modified...
Philip Morris International’s Revenue and Stock Jumps Due to IQOS
Philip Morris International (PMI) can thank the emerging market of heat-not-burn for not only meeting but beating recent revenue estimates.
Earlier this year, the U.S....