
HURDLES AHEAD FOR MIXERS
Now for the bad news: As most in the industry are
well aware, the deeming regulations place numerous
hurdles in the path of manufacturers of the newly
regulated products, from submitting ingredient lists
and registering manufacturing facilities to undergo-
ing one of two onerous and prohibitively expensive
product approval application processes to enable
products introduced to the market over the last nine
years to stay on store shelves. For most retailers,
the biggest impact—and it’s a significant one—of
these requirements will be a dramatic reduction in
the number of products available in the market. As-
suming the regulations remain in effect despite the
mounting legal challenges the agency faces, the field
will narrow considerably over the compliance period
as manufacturers who cannot afford the costly pro-
cess fold their tents.
However, retailers who have been blending pipe
tobacco or mixing e-liquid face a more pressing
concern, notes Briant. “There is a new require-
ment under the deeming regulations that requires
retailers who formulate new flavors of pipe tobacco
by blending tobaccos together and/or who mix e-
liquid nicotine flavors together to form new flavors
to be regulated as manufacturers,” he explains. This
would mean retailers would need to register with
the FDA by December 31, 2016; file a list of pipe
tobacco blends they make and an ingredient list for
each one, and possibly complete and file pre-market
approval applications. “The cost to comply with the
manufacturing regulations…could result in these
retailers ceasing to blend pipe tobacco or e-liquids.”
(See box, at top p. 34).
In fact, the FDA deeming regulations state that
the agency expects retailers to do just that—quit
blending pipe tobacco or mixing e-liquids. The
trouble is that this will put a great many of retailers
who built a business out of catering to customers
who want their own pipe tobacco or e-liquid blends
out of business. In fact, in the vape shop chan-
nel alone, a significant number of the 8,500 vape
shops that Wells Fargo’s Bonnie Herzog estimates
are operating in the U.S. market would be likely to
disappear in the deeming regulations’ wake if that
provision stands.
Even vape shops that don’t blend their own e-liq-
uids will struggle, since the range of products avail-
Actions by Altria
As the lawsuits challenging FDA’s deeming regulations continue to
pile up, Altria can already count one small victory. The company was
one of the first to take action, filing a lawsuit in defense of its John
Middleton Company Black & Mild cigar brand. Among the many re-
strictions that now apply to newly regulated products is a ban on use
of words like “light” and “mild” as descriptors. However, Altria argued
that this rule violates the First Amendment that protects trademarks
and brand names, in addition to the Fifth Amendment that prohibits
taking private property for public use without compensation.
FDA was quick to concede the point. In August, the agency in-
formed Altria that it would not enforce the provision, according
to Marty Barrington, CEO of Altria Group. “FDA has informed us
that they do not, at this time, intend to enforce that [provision]
against Black & Mild. In consideration of that, we have withdrawn
our lawsuit,” he says. “The parties have reserved their rights, but
we will continue to use Black & Mild unless something changes.”
However, the company is still engaging with FDA on multiple
fronts, among them its plans for e-cigarette subsidiary NuMark
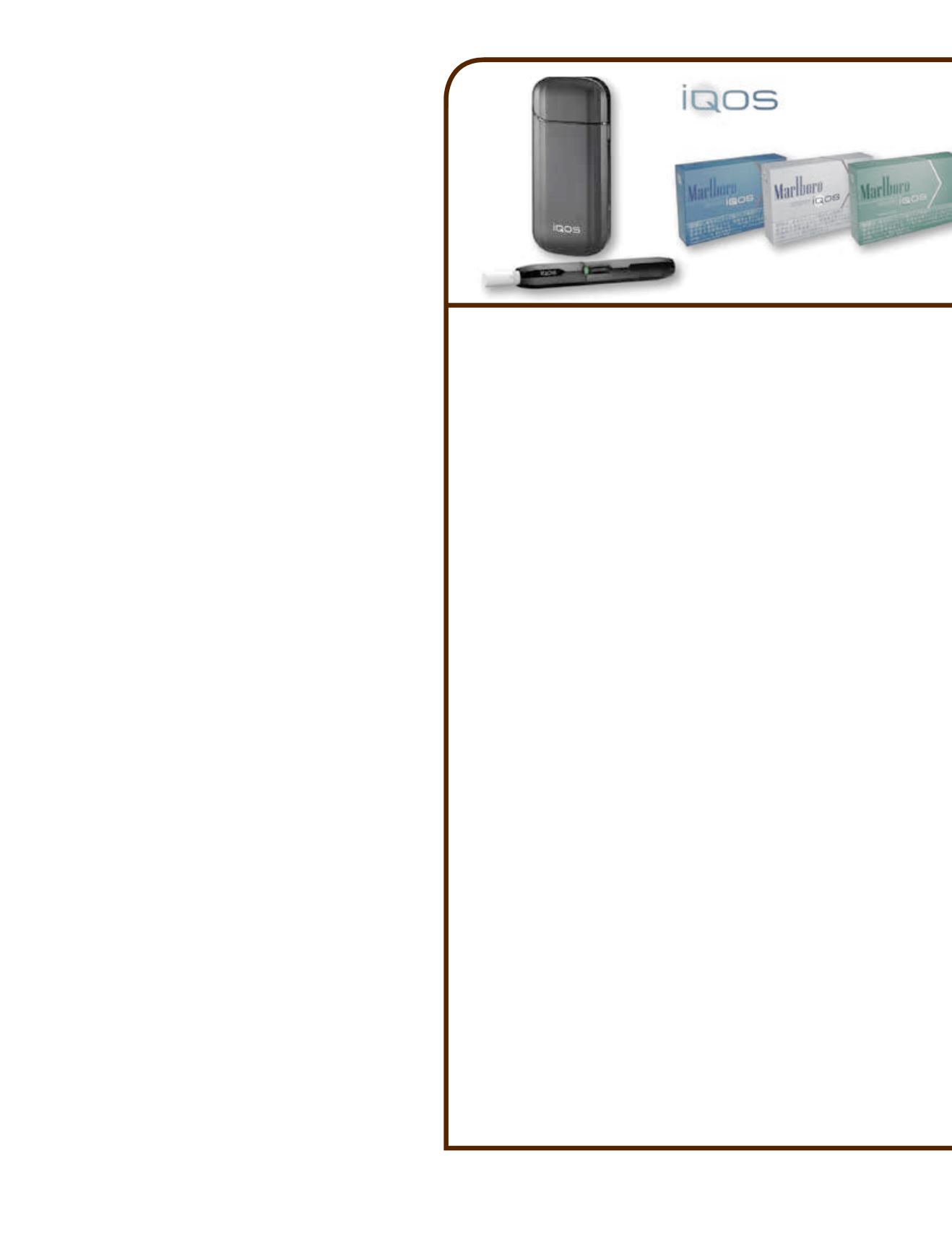
(maker of MarkTen) and PMI’s IQOS heat-not-burn product. “On
the heated tobacco platform, our work with [PMI] in its FDA appli-
cations for pre-market approval authorization and a modified-risk
tobacco product designation remains on plan,” Barrington says.
“We are making excellent progress on commercialization plans for
the U.S. market.”
In the classic scenario of misery making strange bedfellows,
Altria has also joined the vapor business community—made up
in large part by smaller companies looking to lure away smokers
loyal to PMI brands—in protesting the deeming rule’s February 15,
2007 grandfather date. A month after the regulations were re-
leased, Altria sent 22 pages of comments to FDA making the case
that what it calls “electronic nicotine delivery devices” (ENDS) are
exactly the type of reduced-risk products that Congress intended
for the agency to support “when it empowered FDA to regulate
tobacco products” to improve public health.
“If adopted in its current form, the draft guidance may result
in many existing ENDS being forced off the market and make it
difficult for some manufacturers to develop new ENDS products,”
Altria said. “Should this occur, adult tobacco consumers will be
deprived of important product choices.” As a result, almost all
e-cigarette and vapor products would have to retroactively go
through tougher regulatory requirements to prove that they don’t
cause public harm or entice nonsmokers to consume those prod-
ucts, the report added.
“We are both complying with the regulations and we are try-
ing to influence and advocate where we think [they] can be im-
proved,” says Barrington.
32
TOBACCO BUSINESS INTERNATIONAL
SEPTEMBER/OCTOBER 2016
















